Abstract
INTRO: Immune profiles and immune reconstitution are increasingly studied as important contributors to the prognosis and treatment responses of hematologic malignancy patients. Data on epidemiologic factors influencing immune profiles in hematologic malignancy patients are lacking.
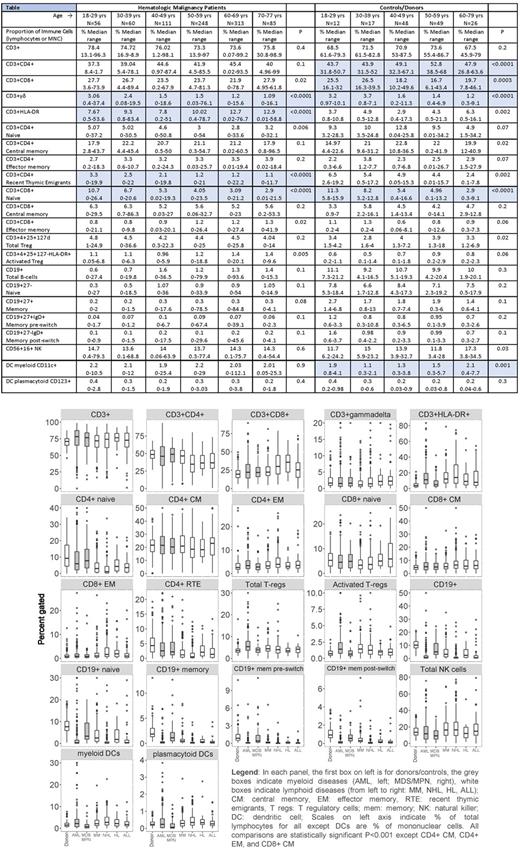
METHODS: We performed flow cytometric analyses of immune panels including T-cell, B-cell, NK cell and dendritic cell (DC) subsets in 1,025 consecutive adult hematologic malignancy patients (N=873) and controls/donors (N=152) between 2006-2016. Immune panels were analyzed on fresh peripheral blood samples drawn during workup before autologous or allogeneic hematopoietic cell transplant (HCT). Hematologic malignancy diagnoses were AML (N=235), MM (N=228), NHL (N=197), MDS/MPN (N=85), ALL (N=60), HL (N=33) and other leukemias (N=35). Patients were 58% male, median age 58 years (range 18-77), 91% non-Hispanic white (NHW), 6% African-American (AA), 1.3% Hispanic, 1.5% other. The HCT-comorbidity index (HCT-CI) in patients showed: 63% with > 1 co-morbidity; 26% moderate pulmonary, 22% psychologic (requiring therapy/counseling), 16% severe pulmonary, 12% cardiac (CHF/CAD/MI), 11% diabetes requiring insulin, 9% obese (BMI>35mg/kg 2), 9% prior cancer. Controls were related apheresis or marrow donors of allogeneic HCT patients: 51% male, median age 49 years (range 19-73), 92% NHW, 4% AA, 1.3% Hispanic, 2.6% other. HCT-CI scoring of controls: 45% with > 1 comorbidity, 20% obese, 20% psychologic, 10% mild liver disease, 7% diabetes. Due to inter-individual cell count variability, immune cells were normalized as percent gated of lymphocytes except DC populations which were gated on mononuclear cells, both on forward and side scatter. To control for multiple comparisons, Bonferroni corrected statistically significant P was set at <0.001.
RESULTS: In controls, males had a significantly lower proportion of CD3+ cells/µl than females (68% vs 73%, P=0.001), NHW had a higher proportion of CD8+ central memory (CM) cells than other race/ethnicities (4.6 vs 2.7%, P=0.004) with no other significant differences by sex or race, although some of the race groups were low in our cohort. In contrast, among hematologic malignancy patients, males had significantly lower CD4+, CD4+ naïve, CD4+ recent thymic emigrants (RTEs), total T-regulatory cells (Treg), and CD19+ naïve cells but significantly higher CD8+ CM and effector memory (EM) cells than females. In addition, NHW had higher CD8+ CM than AA, Hispanic and Other races. Significant differences by age in patients and controls are shown in the Table. Across all age groups, patients had higher proportions of CD3+ cells, lower proportions of B-cells and no difference in NK or DCs than controls. As controls increased in age, CD4+ total significantly increased, while CD8+total, CD8+naive, T-γδ cells decreased, and myeloid DCs were highest at each end of the age spectrum. As patients increased in age, activated HLA-DR T-cells significantly increased, while T-γδ, CD8+naïve, and RTEs significantly decreased. The CD4:8 ratio increased while the CD4+ and CD8+ naïve:EM ratio decreased with age in both controls and patients, however patients had lower CD4:CD8 and naïve:EM ratios than controls. Immunophenotypes by patient disease are shown in the Figure. In general, patients with lymphoid diseases had lower CD3+CD4+ but higher CD3+CD8+, NK and DCs. In controls/donors, there were no significant differences in immune cell profiles by the presence or absence of comorbidities: obesity, diabetes, psychologic and mild liver disease. In patients, T-γδ were significantly lower in patients with diabetes, with no other significant differences for cardiac, psychologic, prior cancer, obesity or pulmonary co-morbidities.
CONCLUSIONS: Additional analyses are ongoing to investigate the influence of prior therapies (chemotherapy, hypomethylating agents, monoclonal antibodies, etc.) and cytogenetic risk groups within each disease (AML, ALL, MDS/MPN, MM, NHL) on immune cell profiles. Studies of immunophenotyping in hematologic malignancies should include adjustment for confounders such as age, sex and race as biologic variables, as well as consideration of the diseases and treatments given. Interestingly, common co-morbidities did not broadly influence immune cell profiles in our cohort of hematologic malignancy patients.
Chen: Actinium Pharmaceuticals: Other: Principal Investigator, SIERRA Trial, Actinium. Hillengass: Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Beijing Medical Award Foundation: Speakers Bureau; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Beijing Life Oasis Public Service Center: Speakers Bureau; Skyline: Membership on an entity's Board of Directors or advisory committees; Curio Science: Speakers Bureau; Adaptive: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Oncotracker: Membership on an entity's Board of Directors or advisory committees; Axxess Network: Membership on an entity's Board of Directors or advisory committees. Wang: Genentech: Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy, Speakers Bureau; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Other: Advisory Board; Mana Therapeutics: Consultancy, Honoraria; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kura Oncology: Consultancy, Honoraria, Other: Advisory board, steering committee, Speakers Bureau; GlaxoSmithKline: Consultancy, Honoraria, Other: Advisory Board; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Kite Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Pfizer: Consultancy, Honoraria, Other: Advisory Board, Speakers Bureau; Stemline Therapeutics: Consultancy, Honoraria, Other: Advisory board, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: Advisory board; Rafael Pharmaceuticals: Other: Data safety monitoring committee; Gilead: Consultancy, Honoraria, Other: Advisory board; Daiichi Sankyo: Consultancy, Honoraria, Other: Advisory board; PTC Therapeutics: Consultancy, Honoraria, Other: Advisory board; Genentech: Consultancy; MacroGenics: Consultancy. Griffiths: Takeda Oncology: Consultancy, Honoraria; Alexion Pharmaceuticals: Consultancy, Research Funding; Apellis Pharmaceuticals: Research Funding; Abbvie: Consultancy, Honoraria; Astex Pharmaceuticals: Honoraria, Research Funding; Genentech: Research Funding; Taiho Oncology: Consultancy, Honoraria; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Novartis: Honoraria; Boston Biomedical: Consultancy. Torka: TG Therapeutics: Membership on an entity's Board of Directors or advisory committees. McCarthy: Bluebird: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Magenta Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Juno: Honoraria, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal